400 blows do not raise hell after all: This month of May 2013 will be remembered as the time when we passed that 400ppm line of CO2 concentration in the atmosphere. The media often uses the crossing of such a round, numeric Rubicons to begin global campaigns and to instigate the enforcement of serious repair measures, but it seems unlikely that is going to be the case with Ocean Acidification for the time being. As frightening as it may sound, if you are in your 30s you might very well live long enough to witness how we also tackle 500. And that will be a very different world, one with even more painfully visible destruction and well recorded examples of irreparable damage done to our oceans.
These past couple weeks have once more produced valuable news and videos about the topic of our documentary and the recurring theme in this blog, the plight of Ocean Acidification:
≈≈The expected 400ppm mark in the news:
A Guardian piece from back in April
CNN writes about first hitting the 400ppm mark at Mauna Loa on May 9th
The Keeling Curve, to get the latest reading (399.91 today)
≈≈Top-notch HD video by the Arctic Monitoring and Assessment Programme (a part of the Arctic Council) about Arctic Ocean Acidification:
More videos by AMAP HERE
≈≈According to a study conducted in Papua New Guinea by a team of scientists from the Australian Institute of Marine Science Ocean Acidification could lead to the extinction of an entire class of marine organisms by 2100:
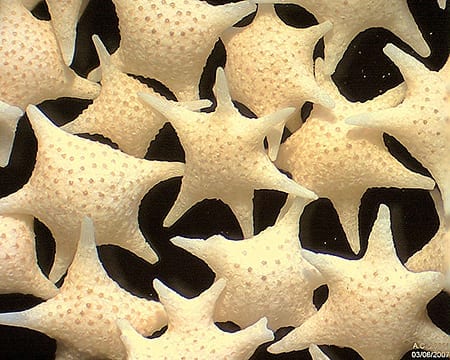
“Forams – or foraminifera – are much like an amoeba with a shell,” explains Dr Sven Uthicke, lead author of the study which was published last week in the prestigious scientific journal Scientific Reports, an online journal of Nature. “As CO2 levels increase, our oceans will become more acidic, making it more difficult for these small marine creatures to form the shells they need to survive.”
Continue reading HERE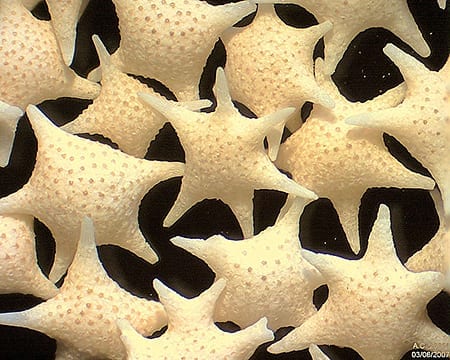
PHOTO: Foraminifera “Star sand” Hatoma Island – Japan
≈≈Video: “In Washington State Ocean Acidification is about People” (by the Ocean Conservacy):
≈≈TED Talk by NOAA’s Shalling Busch under the title: “Ocean Acidification in Washington State”:
≈≈Richard Feely (NOAA) talks about Ocean Acidification at the University of California, Irvine:
≈≈“Shipping pollution along major trade lanes can rival carbon emissions in contributing to the increased acidity of the ocean, according to a new study by an international team.”
In the first global analysis of its kind researchers from the University of Gothenburg, Chalmers University of Technology, the University of Delaware, and the Institute for Advanced Sustainability Studies discovered that during the summer months Ocean Acidification from shipping can equal that from CO2. Gasses such as sulfur and nitrogen oxide, present in the exhaust gases from ships’ engines, can also cause acidification.
“Global shipping has emitted acidifying compounds for decades without emissions controls,” says James J. Corbett, professor of marine policy in the University of Delaware’s College of Earth, Ocean, and Environment. “Only recently have regulatory standards set limits on ship emissions that will take effect between now and 2025.”
“These oxides contribute to long-range pollutant transport, but significant amounts can be deposited within a few hundred nautical miles of the shipping lanes. This study has assessed the consequences of releases from shipping on a monthly basis during one year.
The results show that the greatest acidification from shipping occurs in the northern hemisphere in coastal areas during the summer. In addition, acidification occurs in open-ocean regions surrounding heavily trafficked shipping lanes.”
SOURCE
≈≈Turns out there actually is a Tums for acidic waters, at least on a local level: Oysters. Not only have these mollusks fed coastal communities for millennia, filtered and cleaned water while providing habitat for their own young and for other species, but there was actually a time when they were significantly responsible for buffering the increasing acidity of ocean waters.
“Ecosystem effects of shell aggregations and cycling in coastal waters: An example of Chesapeake Bay oyster reefs,” a study co-authored by Professor Roger Mann of the Virginia Institute of Marine Science, George Waldbusser of Oregon State University and Eric Powell of the Haskin Shellfish Research Laboratory at Rutgers University, suggests that in 1870—before people began large-scale harvesting of oyster meat and shells from the Chesapeake—the amount of oyster shell exposed to Bay waters was more than 100 times greater than today, with an equally enhanced capacity to buffer acidity.
“Our data show that that oyster reefs likely played a key role in the pH budget of pre-harvest Chesapeake Bay,” says Mann. “The amount of carbonate in the shells of living oysters at that time was roughly equal to the total amount of carbonate dissolved in the modern Bay. If similar numbers of oysters were alive today, they could take up about half of the carbonate that rivers currently carry into Bay waters.”
Read more about this fascinating news HERE
≈≈Computer animated video by AFP (Agence France-Presse) on Ocean Acidification:
≈≈Dr. Chris Gobler (Stony Brook University) talks about Ocean Acidification: